Learning to Make Soap: Part 5
What makes a good bar of soap?

So I have designed my recipes, made them, let them cure and now I get to test them - but what actually does a good bar of soap look like? How am I going to know if I have got it right? That this is the recipe?
You would think that I might have thought about this sooner, and in a way I have as I designed the Small Kindness soaps using oils that will give the soap it's specific qualities. But now I have actually made them and got to use them, what is it I am actually looking for? A good lather? Moisturising? And what does that actually mean?
I am planning on sending these first Small Kindness soaps to my parents to test. Will what they think makes a good soap be different to me? Will they get put off by one of the scents, or when they say it has a good lather, is that really the same as what I think a good lather is? Under normal circumstances I would have delivered my soaps in person and tried them with my parents. But at the moment we are still in a national lockdown in England and I am unable to see them, which is completely rubbish (and not just because of the soap!! Love you Mum and Dad ❤).
Ok so what do I know? Well I definitely know what I don't like in a bar of soap - I don't like bars that go all mushy in the soap dish, or feel really tacky and sticky to touch. I do like a bubbly lather, but I definitely don't want the soap to be drying. So I would like a mild bar of soap. And what I haven't mentioned is cleansing - the whole point of the bar is that it cleans you. So should that be the most important? Or should it be the bubbles? Or the conditioning?
If you use a soap calculator it will generally give you a "score" for the following soap properties
Hardness – the firmness of the soap bar
Cleansing – the ability of the soap to trap dirt and wash it away
Conditioning – the amount of moisture that is left on the skin
Bubbly – the soap’s ability to lather up and get bubbly
Creamy – the stability and creaminess of the lather
Looking at this list you would say that you want your bar of soap to be all these things, but, as with most things in life, it is a bit of a balancing act. The more cleansing the soap the more it will dry and irritate the skin, the more creamy and stable your lather the less bubbly it will be. But at least these are general qualities my Mum and Dad can look for and "score" when testing my soap.
Okay. So if that isn't enough there is one more element I need to consider when asking my parents to test my soaps. I live in Birmingham which is a soft water area and my parents live near Worthing, on the South coast of the UK, and they have hard water. Well that is super interesting (!) but what does that mean and why does it matter? Water hardness is a measure of the level of mineral components in your water, such as magnesium and calcium and water hardness can effect the cleaning ability of soaps!
Great! So how does that work? If you remember back to Learning to Make Soap: Part 2, soap is a fatty acid salt and in solid soap this is a sodium salt. So for instance my soaps contain olive oil, which contains a number of fatty acids, but the most predominant one is oleic acid. So my soaps contain sodium oleate. However, when a soap is used in hard water the calcium and magnesium ions in the hard water react with with the fatty acid salt (in this example sodium oleate) to result in the formation of the calcium and magnesium salt of the fatty acid (magnesium oleate and calcium oleate). These are insoluble substances that make up soap scum which limits the soaps cleaning ability and leave a tacky or greasy residue.
So although the soaps I have designed work well in Birmingham there is a high possibility that they may not be so great in a hard water area! So luckily my lovely parents will be able to test this for me ❤. But what if they don't work - is there anything I can do?
Thankfully there is - I can add a chelator - this will capture the magnesium and calcium ions from the hard water and prevent it from forming the magnesium or calcium fatty acid salt or soap scum. Phew!
There are a number of options, but I think for Small Kindness soaps the best option would be to use citric acid. Actually citric acid isn't a chelator, but it reacts with sodium hydroxide to make sodium citrate, which is a chelator. To be honest I could buy sodium citrate and add it directly into my soaps, but citric acid is a readily available, fairly cheap ingredient that is manufactured from the fermentation of sugars. Another chelator that is often added to soaps is EDTA - I personally do not want to add this to my soaps because EDTA is poorly biodegradable and has been found to be one of the most common pollutants found in surface waters in central Europe (1).
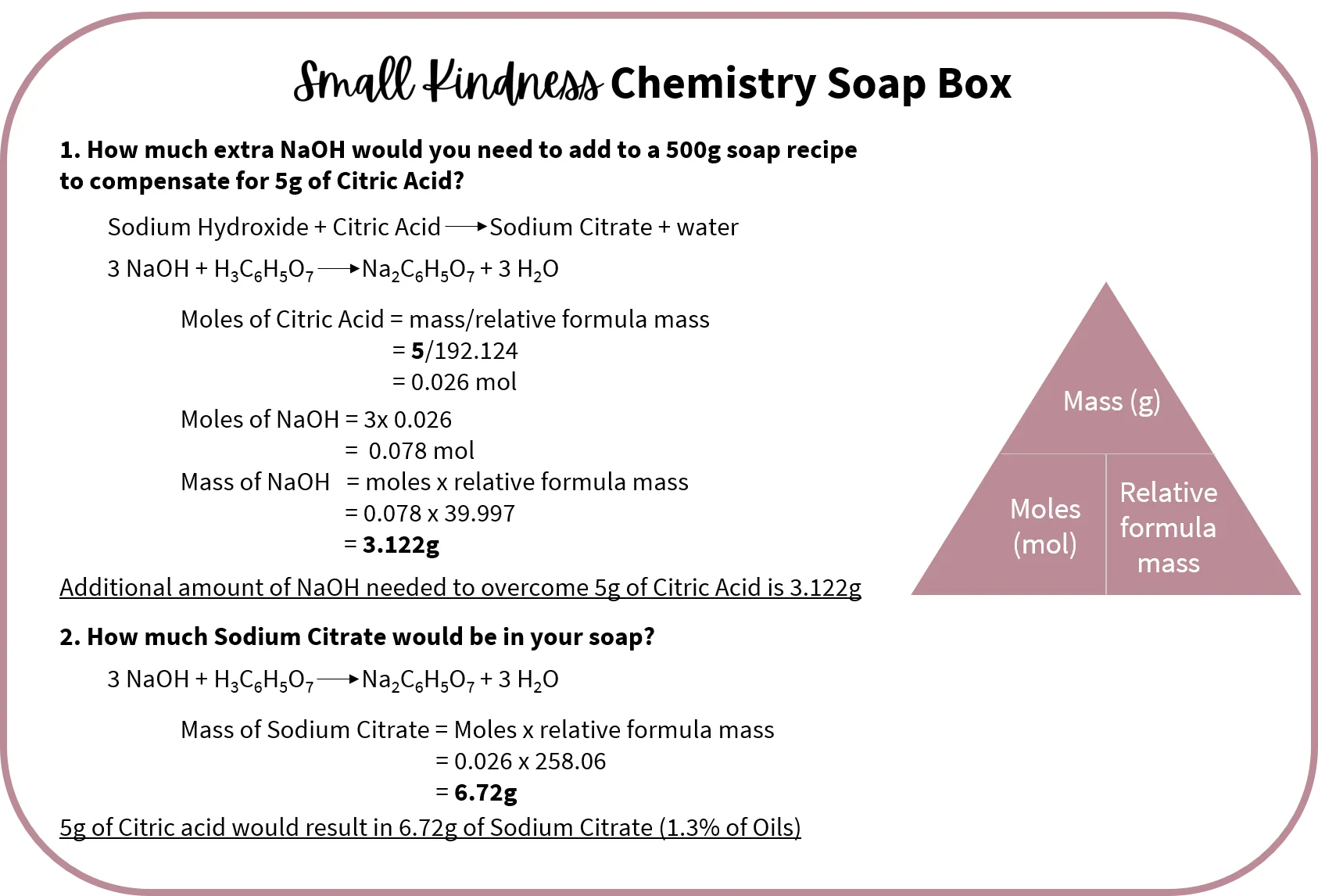
So what do I have to consider if I need to add citric acid to my soaps to help overcome the effect of the hard water? Well, as mentioned above, citric acid reacts with sodium hydroxide to form sodium citrate and water. This means it is actually using up some of the sodium hydroxide. If this isn't compensated for by adding additional sodium hydroxide it will result in a much higher percentage of superfat in my soap than I had originally intended. This might result in a tacky soap - one of the things I definitely do not want.
The suggested usage rate of citric acid is 1-2% per total soaping oils. So if I am making a 500g test batch and I want a rate of 1% that would mean adding 5g of citric acid. To make sure this doesn't effect the superfat amount how much additional NaOH do I need to add? If you are interested I have shown how to calculate this in the box below (and how much sodium citrate you would have in this batch). But in short you would need to add an extra 3.1g of NaOH for every 5g of citric acid you add to your recipe.
Sorry I seem to have gone off on a small chemistry tangent - let's get back to my original question - what makes a good soap?
I really do think this is personal preference. The whole purpose of soap is to clean, so it definitely needs to do that, but it needs to be mild too, meaning it is not so cleansing that it actually ends up drying and irritating the skin. So plenty of bubbles that leaves your skin feeling moisturised - but not tacky! Oh and the bar definitely shouldn't go mushy!
So what do I ask my parents to measure? I think the most important element is with regards the hard water - does the soap actually clean them? Do they get a lather at all? Does it feel sticky, does it go mushy in the soap dish? But most importantly do they actually like it, do they get any kind of enjoyment out of using it? Would they want to buy it? (not that they ever would have to!).
I want to make soaps that are a joy to use, that make your morning shower feel like a treat and are kind to your skin, people and the planet!
Right I am off to post my soaps to my parents! I will be back next week, but for now stay safe xx
References 1. Oviedo, Claudia, & Rodríguez, Jaime. (2003). EDTA: the chelating agent under environmental scrutiny. Química Nova, 26(6), 901-905. https://doi.org/10.1590/S0100-40422003000600020
...
I am Kelly Townsend and this is the Small Kindness Blog. I am a scientist, a bee lover, a rewilding obsessive, and I want to spread Small Kindnesses through the medium of soap. Follow me on Facebook, Instagram and Twitter for your daily dose of kindness (as well as to see how the soap making is going!)
